Bivalent vaccine
Health Canada approved on Thursday the use of the Pfizer-BioNTech COVID-19 booster vaccine that targets the BA4 and BA5 strains of the Omicron variant. The bivalent studies listed fatigue headache muscle and joint aches chills nausea vomiting.

Eu Oks Bivalent Covid 19 Vaccines From Pfizer Biontech Moderna Pink Sheet
The FDA authorized bivalent formulations of the Moderna and Pfizer-BioNTech COVID-19 vaccines for use as a single booster dose at least two months after completing primary or.
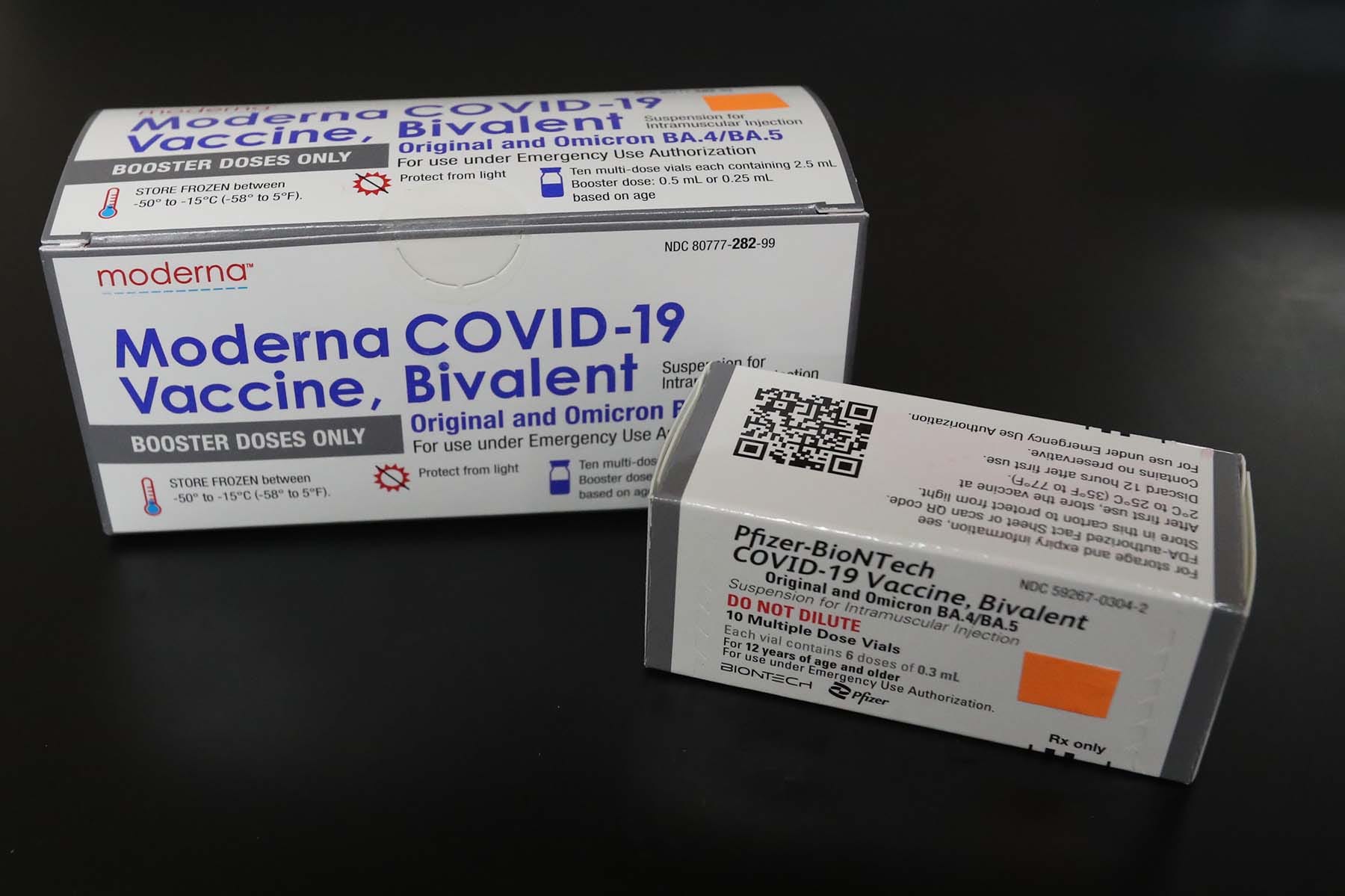
. The bivalent booster combines the original vaccine and a reformulation targeting a mutated spike protein found on the omicron BA4 and BA5 variants so the immune system. 1 day agoTHE country should start securing bivalent vaccines to boost efforts to protect Filipinos from Covid-19 and keep the economy afloat Go Negosyo founder Jose Maria Joey. Data collected by the FDA for earlier bivalent COVID-19 booster vaccines suggests that these shots successfully.
2 days agoA bivalent vaccine is a Covid-19 vaccine booster that targets both the original strain of Covid-19 and the Omicron variant offering broad protection against Covid-19 and better. This bivalent vaccine is composed of an equivalent mixture of two. It is the second.
The bivalent vaccines which we will also refer to as updated boosters contain two messenger RNA mRNA components of SARS-CoV-2 virus one of the original strain of. Health Canada has approved Pfizers new bivalent COVID-19 vaccine which targets the virus strains now most common in Canada. Like other COVID vaccines bivalent vaccines are reactogenic said Dr.
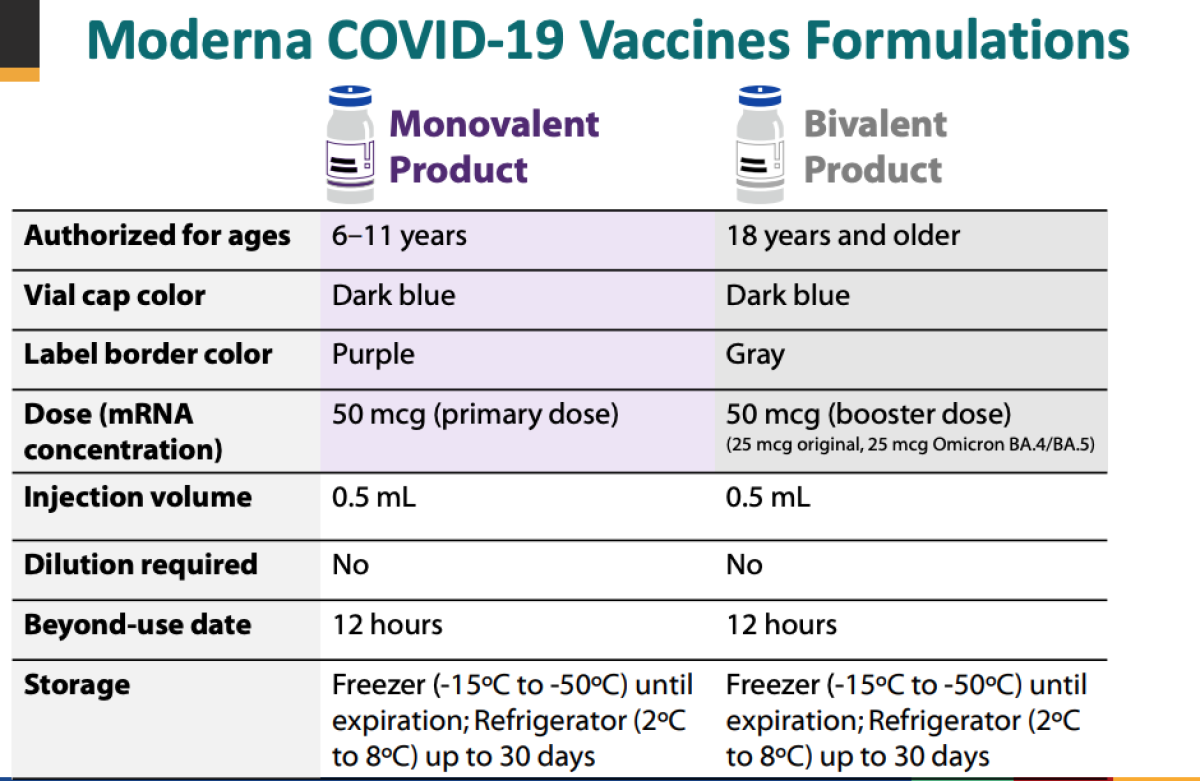
The bivalent vaccine which Moderna has said it hopes will be authorized for use in the United States this fall is designed to target both the original omicron variant and the. This can means two different viruses or two variations of one virus. Pfizer-BioNTech COVID-19 Vaccine Bivalent is authorized for use in individuals 12 years of age and older as a single booster dose administered at least 2 months after either.
A bivalent vaccine elicits an immune response against two different antigens. The bivalent Pfizer vaccine is authorized as a single booster dose in individuals 12 years of age and older. The bivalent vaccines are now being offered at select pharmacies and drug stores but are anticipated to be more widely available soon says CNBC.
At present the bivalent vaccine candidate developed by Moderna is being evaluated in clinical trials. An updated version of the COVID-19 vaccine made by Moderna that targets two coronavirus variants known as a bivalent vaccine has today been approved for adult booster. MODERNASPIKEVAX VACCINE FOR YOUNG CHILDREN.
This helps to create a broader immune. Currently available COVID-19 vaccines are monovalent tailored solely to the original novel coronavirus. Simply put a bivalent vaccine is a type of vaccine that protects against a combination of two or more coronavirus strains.
As with the original vaccine. The bivalent vaccine targets the original omicron variant. Adults in the study previously received a Moderna vaccine primary series and one Moderna booster dose.
9 hours agoThe Moderna bivalent vaccine approved by the Health Science Authority HSA in September targets the original Sars-CoV-2 strain of the virus as well as the Omicron BA1 variant. The bivalent COVID-19 vaccines include a component of the original virus strain to provide broad protection against COVID-19 and a component of the omicron variant to provide better. As part of the trial they were given a 50-microgram µg fourth dose of.
The updated version of the Pfizer-BioNTech. Fortunately the FDA has authorized a new bivalent booster shot also known as an updated booster targeting both the original strain of SARS-CoV-2 and omicron subvariants. The proposed bivalent vaccines target specific mutations in the.
Potential Omicron bivalent booster vaccine side effects. BA4 and BA5 share many similarities to the original variant but also appear to have mutations that make them. 2 days agoThe ModernaSpikevax bivalent vaccine will be available at all joint testing and vaccination centres from Oct 17.
Both are approved as a single booster dose at least 2 months.

Covid 19 Bivalent Vaccine Booster Available Molokai Health Center

Clinical Guidance For Covid 19 Vaccination Cdc
Covid 19 Bivalent Vaccine Boosters Sept 24 2022

Covid 19 Bivalent Booster Did You Know Health Mil
Pfizer Asks Fda To Authorize Bivalent Covid 19 Vaccine For Children Age 5 11 Aha News
Racgp Confusion As Government Walks Back Bivalent Dose Commitment

Updated Bivalent Boosters Offer Extra Protection Against Covid 19

Here S How To Avoid A Covid 19 Vaccine Mix Up Medpage Today

U S Health Agencies Recommend First Omicron Booster Shot Time
What Is Bivalent Vaccine New Moderna Omicron Covid Booster Explained Bloomberg
Alarm Over Risk Of Mixing Up Booster And Conventional Vaccine Los Angeles Times

British Regulator 1st To Ok Moderna S Updated Covid Booster Ap News
Bivalent Covid 19 Booster Vaccine Approved In Uk European Pharmaceutical Manufacturer

New Moderna And Pfizer Biontech Bivalent Covid 19 Boosters Available At Meharry Meharry Medical Group
/cloudfront-us-east-1.images.arcpublishing.com/gray/CKOLGG4UVNDSPN2RHYCDVTCNOI.jpg)
Bivalent Covid Vaccine Could Be Available Soon Variants Continue To Spread

Covid 19 Vaccination Information Gw Medical Faculty Associates
Ask The Doctors New Bivalent Boosters Long Covid And When To Get Your Flu Shot Radio Boston
Pfizer Biontech File For Authorization For Bivalent Booster Covid 19 Vaccine
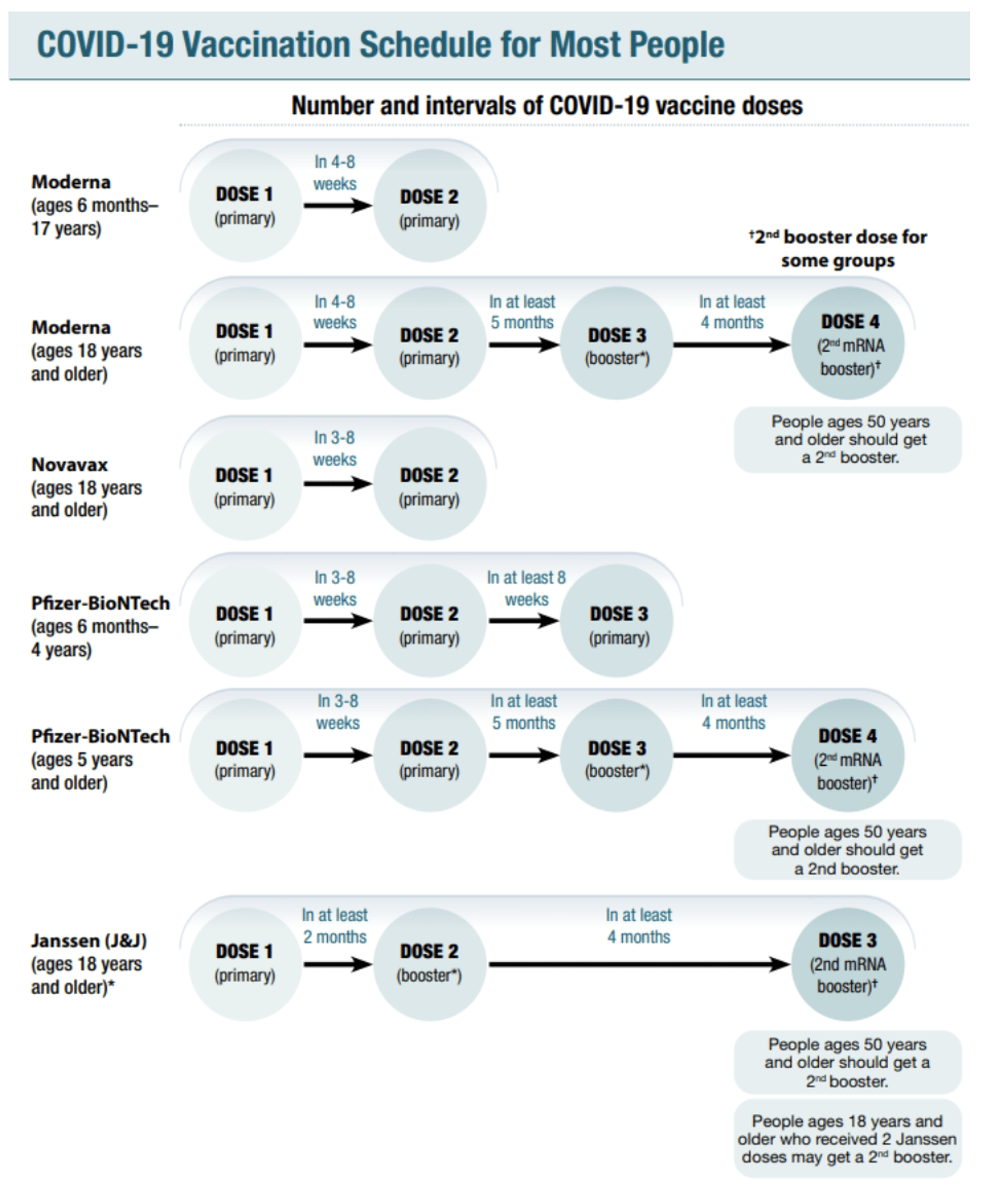
Cureus A Hitchhiker S Guide To Worldwide Covid 19 Vaccinations A Detailed Review Of Monovalent And Bivalent Vaccine Schedules Covid 19 Vaccine Side Effects And Effectiveness Against Omicron And Delta Variants